Medicinal Products across Indications and Geographies
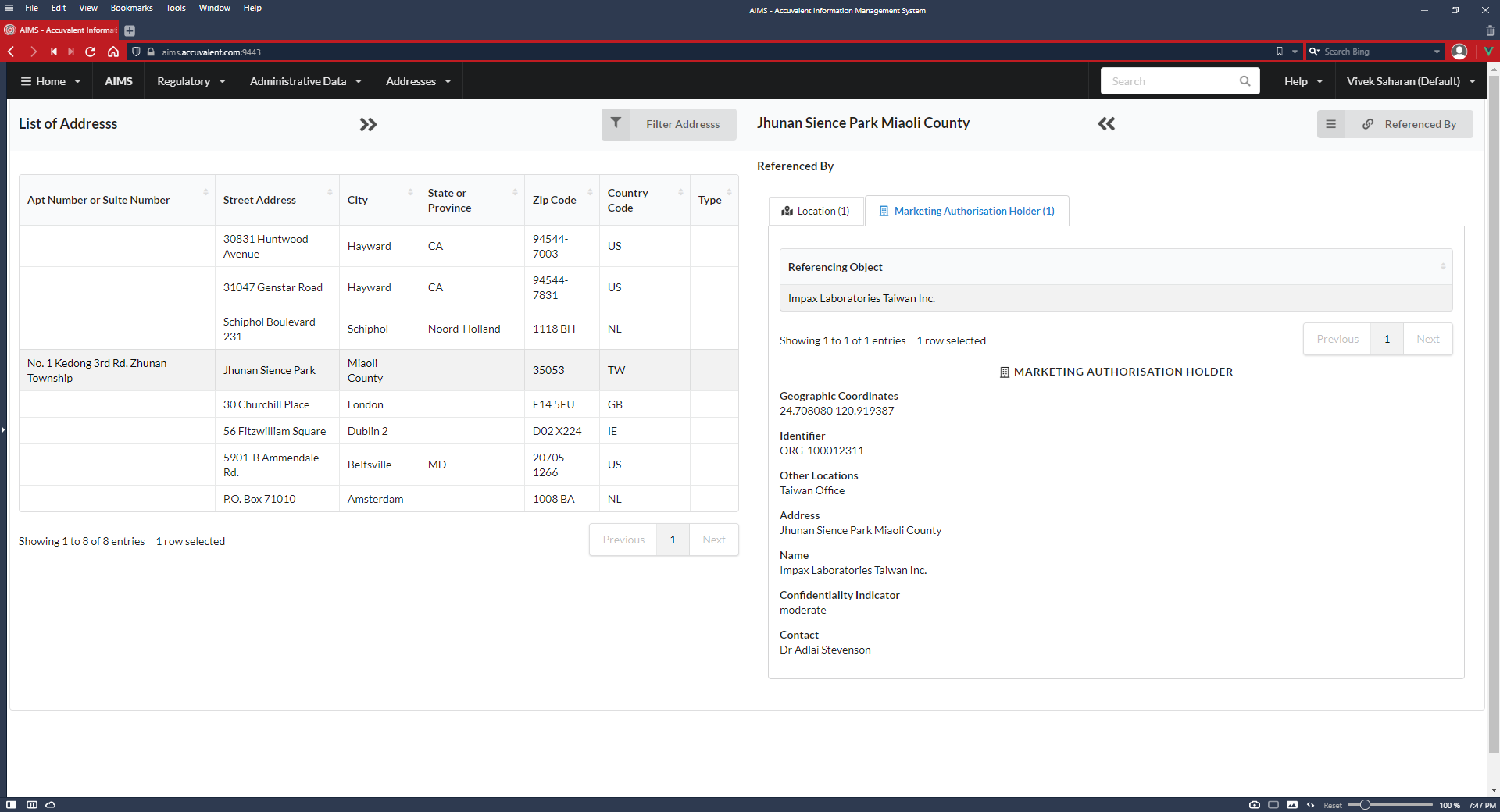
Maintain consistent product identification, coding and documentation. Easily exchange medicinal product information with global regulators, manufacturers, suppliers and distributors.
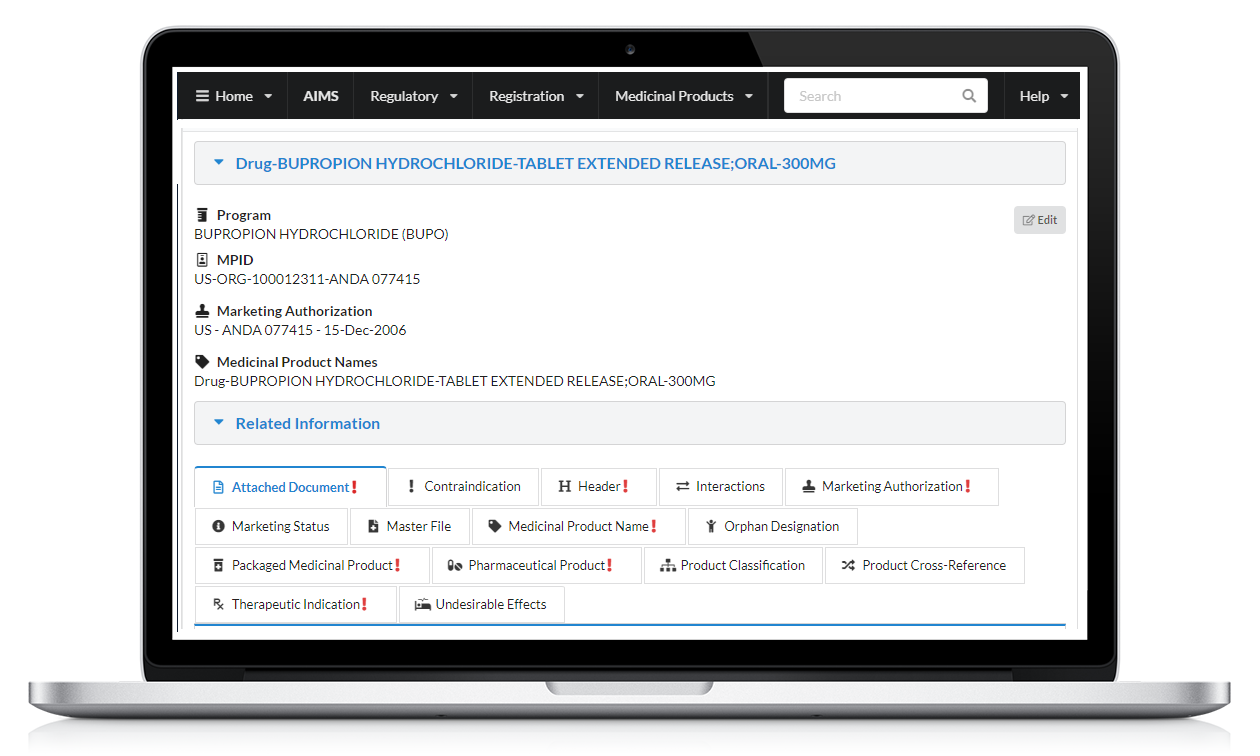
Clinical Particulars including therapeutic indications, contraindications, interactions, undesirable effects
Marketing Authorizations with marketing status, medicinal product names, cross-references, master files, orphan designations
Packaged Medicinal Products including description of all packaging, containers, manufactured product and its ingredients
Pharmaceutical Product with substance, dosage form, route of administration, product classifications
Manufacturer/Establishment, regulated documents by region
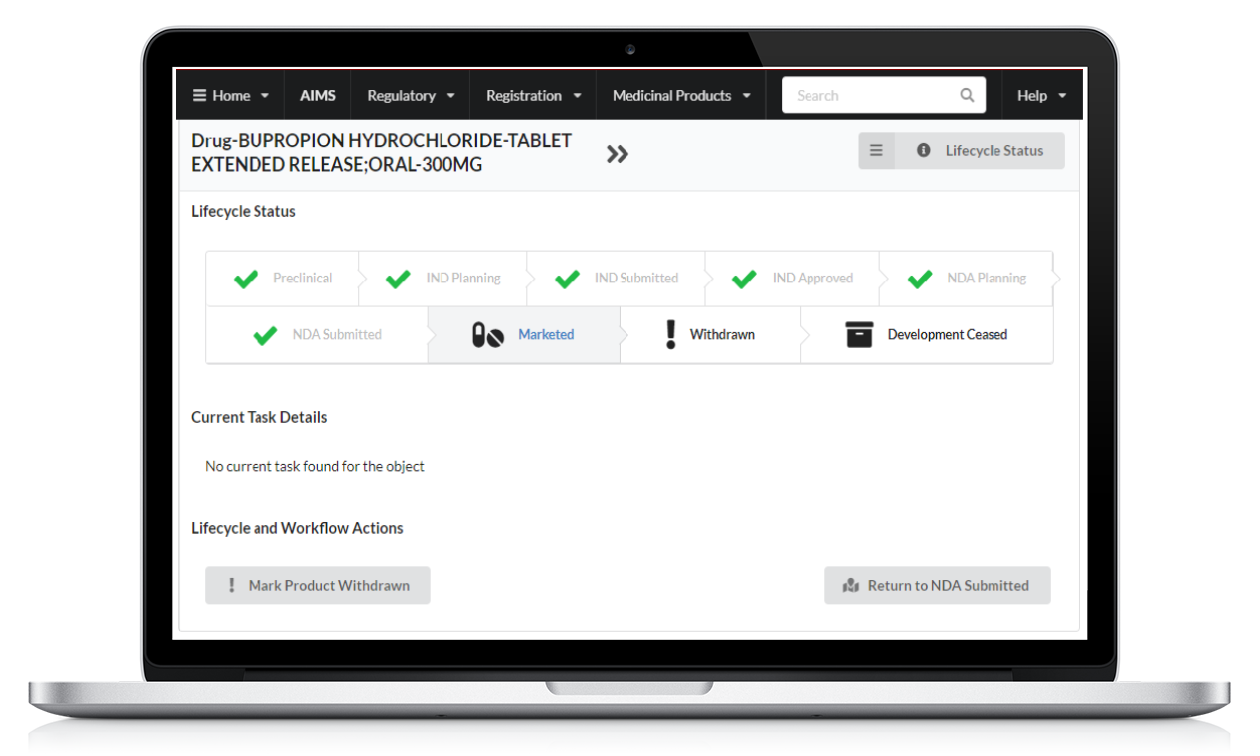
Easily structure medicinal product lifecycle tasks and utilize resources. Improve chances of successful development and minimize risks through the development process.
Clinical Particulars including therapeutic indications, contraindications, interactions, undesirable effects
Marketing Authorizations with marketing status, medicinal product names, cross-references, master files, orphan designations
Packaged Medicinal Products including description of all packaging, containers, manufactured product and its ingredients
Pharmaceutical Product with substance, dosage form, route of administration, product classifications
Manufacturer/Establishment, regulated documents by region