Information at your fingertips
Your information should not live in applications or vaults. You should not have to go search and find the information you need. Rather, the information should be readily available and brought to you – when you need it.
Having data that are consistent, reliable, accessible and well linked is still one of the biggest challenges facing pharmaceutical R&D today. Adopting an information-centric approach, with a focus on making the information available in each functional area and through the whole information life cycle, will greatly facilitate the ability to find, use and share data.
Surfacing not just the immediate information users are working with, but also all related and connected information, allows rapid and effective decision making by permitting users to deeply examine the data.
Read on to learn how AIMS provides you with a window onto the world of your information.
Getting Your Tasks Done
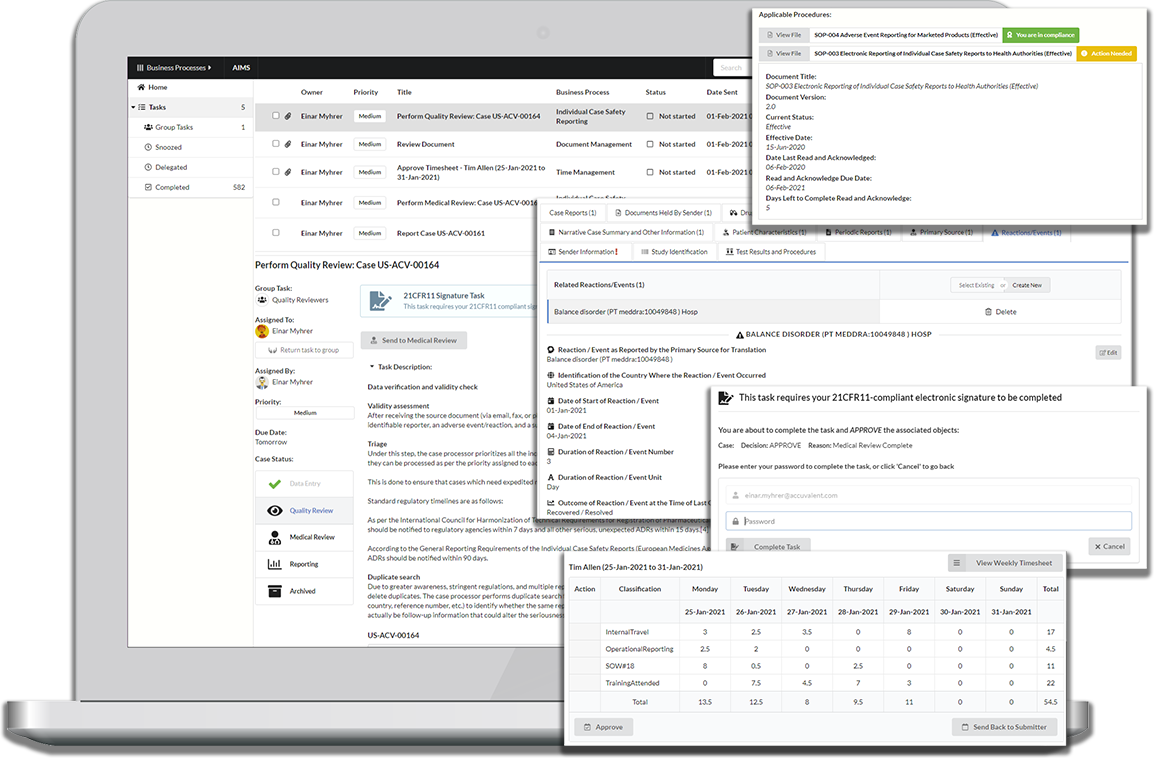
Everything you need to get things done in a single screen
Your information does not live in application or vault-defined siloes, but is seamlessly integrated across all business processes you work with.
When working on tasks a complete view into your information is presented with a tailored experience based on your individual perspective, making it easy to get your tasks done quickly and correctly.
Conversely, we don’t believe you have to go to a task list or inbox to complete your task if we are already working with a piece information. Just as we bring all information to a task, we also bring all tasks to your information.
Features
Full Lifecycle and Workflow visibility
Policy driven assignment of tasks
Tasks can be assigned to individuals or groups
Policy driven delegation of tasks
Automatic delegation of tasks when out of office
Task due dates
Ability to snooze tasks
Assign task priorities
Contextual help with specific instruction for what you are doing now
Embedded approved procedures applicable to what you are doing
Embedded compliance to help you stay current with training curricula
Embedded Compliance
Always see approved procedures, complete training as you work
We bring your current policies and procedures to you – where you need them and when you need them.
By letting you see which procedures apply to the work you are doing right now we minimize the risk of being out of compliance because you didn’t remember what procedures apply. By always showing you the latest approved procedures we minimize the risk of being out of compliance because you are following an outdated procedure.
By clearly showing you whether you need to train on the applicable procedures we make sure you know that your training records are up date. And if you do need to train on a procedure? We let you read and acknowledge the procedures right here and right now, of course. No need to go to a different system to create and enter your training record.

Features
Applicable procedures specific to your current context
Current and effective procedures are always shown.
View procedures directly in the browser
Color coded summary of training compliance
Display procedure status such as effective dates, etc.
Complete Read and Acknowledge directly when viewing procedures
Impact Analysis
Find out where information is used and what impact changes will have
Making quick and efficient decisions many times requires comprehensive views of linked, related or underlying data rather than just the information itself.
By automatically creating and managing semantic relationships between related pieces of information and letting you browse and explore these links in a one of a kind Information Browser, we let you answer questions that have been difficult to answer in the past.
Need to know what eCTD applications and submissions a particular Investigator Brochure is part of, and to which agencies they have been submitted and when? Need to assess impact of an emerging contraindication for a marketed drug? These are insights that are current difficult and time consuming to answer. We help to accelerate finding robust answers to these questions.
To achieve this end-to-end visibility across different functions such as discovery, clinical development, medical affairs and labeling we bring a number of capabilities, including single sources of data and documents, the ability to establish cross-linkages between elements, robust data quality, workflow management, along with robust access controls to ensure that specific information elements are visible only to those who are authorized to see it.
Collaboration
Collaboration is key to bringing drugs to market faster
In recent years, pharma and biotech companies have begun to embrace a more collaborative way of working to help overcome some of the challenges the industry is facing. Expiring patents, rising costs of bringing a drug to market, availability of new technology, and tight regulatory environments are just some of the reasons why they are forming all manner of partnerships, even with competitors.
We enable this growing need for external collaboration by making it easy to securely share information, both within the organization and with external partners and stakeholders, so that you can bring your drugs to market faster and more efficiently.

Path to Meaningful Automation

The benefit of unified visibility across your information
As we start to develop intelligence based on these patterns, the system will start to suggest actions and behaviors. Perhaps a workflow is usually started and routed to a group of people when a certain type of information is uploaded to the system. We can pick up on these patterns and start the process of automating them. As confidence in these predictions grow, some may be completely automated.
Product Security

Secure information in a secure cloud
We treat security as an integral part of our business and with SaaS offerings for large enterprise IT customers it is incredibly important to us for this to be an area of strength. We know that you trust us with very sensitive data, and we strive to protect your data with the utmost care.
Our focus on security cover a broad range of topics including product security, physical office security, employee screening, office network security, business continuity, disaster recovery, data security, secure IT policy, asset security and data center security in order to provide the best overall security for our customers.
Here we focus on product related security topics. These are broken into 3 primary categories of security: operations security, development security and application security.
Pharma R&D Solutions
Discover our solutions for the various functional areas within Parma R&D

